Subhajit Hazra, experienced medical writer on Kolabtree, writes about the role of chloroquine and hydroxychloroquine in the treatment of COVID-19, with summarized data from clinical trials.
In December 2019, China was struck by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or COVID-19. Since then, the disease has claimed more than 1,70,501 deaths globally. (1,2) At present, researchers from across the globe are testing out various drugs to combat this ever-rising incidence of death. In this regard, the present article aims to clarify debates over the use of chloroquine or hydroxychloroquine for the treatment of COVID-19 through an evidenced-based approach.
Role of Chloroquine (CQ) & Hydroxychloroquine (HCQ) As Monotherapy
Currently there are no FDA approved drugs for the treatment of COVID-19. (3) In such a situation researchers are considering repurposing drugs as one of the viable options beyond standard care for the effective management of the disease. Chloroquine and hydroxychloroquine in this respect, have been subjected through a series of trials (4) (Table 1) to understand whether the drug can safely be used in patients affected with COVID-19.
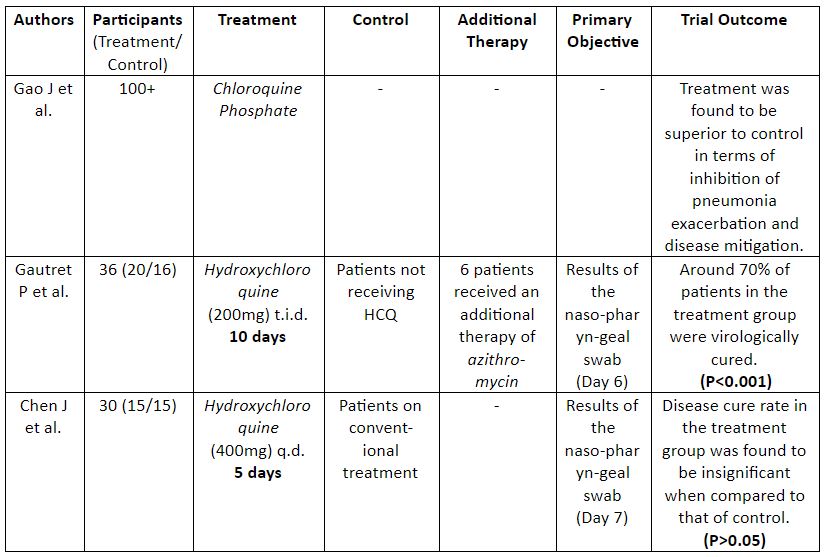 Table 1: Summary Of Clinical Trials On CQ & HCQ
Table 1: Summary Of Clinical Trials On CQ & HCQ
A detailed analysis of the trials showed that these studies were underpowered (according to authors) and lacked long-term follow-up data required to support the role of CQ and HCQ in the treatment of COVID-19. Additionally, the trials suffered from allocation bias as participants were not randomized in the study. Therefore these studies were inconclusive and this demanded the need for further randomized controlled trials for the repurposing of these drugs to their correct effect.
Role of Hydroxychloroquine In Combination Therapy
Azithromycin is known to have been used earlier for the treatment of Zika, Ebola, and severe respiratory tract infections. (5) Considering this, researchers carried out trials to elucidate the effect of combination (HCQ+Azithromycin) therapy on patients affected with COVID-19. However, such trials have shown conflicting results (Table 2) thus, demanding the need to continue the search for other feasible treatment options.
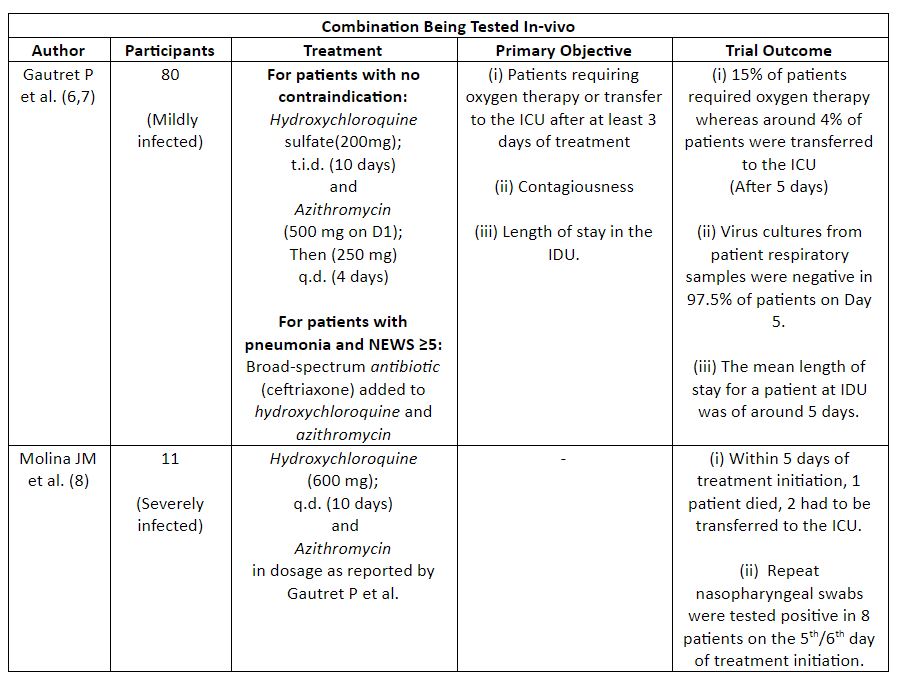 Table 2: Summary Of Trials On Combination Therapy (HCQ and Azithromycin)
Table 2: Summary Of Trials On Combination Therapy (HCQ and Azithromycin)
(NEWS: National Early Warning Score; IDU: Infectious Diseases Ward; ICU: Intensive Care Unit)
Conclusion
The COVID-19 pandemic is ravaging countries around the world. Healthcare experts are trying their best to minimize its casualties on the general population. This compulsion can at times, lead to being inclined towards the more lucrative therapy rather than the one with clear scientific evidence. Therefore, at times such as these, one should only consider therapies based on evidenced-based literature reviews. Thus to conclude, currently, neither hydroxychloroquine nor its combination (of any kind) has been approved by the USFDA or by any regulatory authorities across the world as there is no clear evidence of its efficacy in the treatment of COVID-19.
References
- Singhal T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020, 87: 281–286.
- Worldometer. (21.04.20). Retrieved from https://www.worldometers.info/coronavirus/
- Information for Clinicians on Investigational Therapeutics for Patients with COVID-19. Centers for Disease Control and Prevention. (21.04.20). Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html
- Gbinigie K and Frie K. Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review [published online ahead of print, 2020 Apr 7]. BJGP Open.
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [published online ahead of print, 2020 Mar 20]. Int J Antimicrob Agents. 2020; 105949.
- Vincent JL, Taccone FS. Understanding pathways to death in patients with COVID-19 [published online ahead of print, 2020 Apr 6]. Lancet Respir Med. 2020; S2213-2600(20)30165-X.
- Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020; 101663.
- Molina JM, Delaugerre C, Goff JL, et al. No Evidence of Rapid Antiviral Clearance or Clinical Benefit with the Combination of Hydroxychloroquine and Azithromycin in Patients with Severe COVID-19 Infection [published online ahead of print, 2020 Mar 30]. Med Mal Infect. 2020; S0399-077X (20)30085-8.
Need to consult a coronavirus expert or COVID-19 researcher? View freelance COVID-19 experts on Kolabtree who can help you.