Clinical trial costs have surged over the past decade, with recent reports stating that from 2008 to 2013, the average cost per patient increased by $23,600. A major contributor to the cost of a clinical trial is subject recruitment and the associated staff costs. So, examining every opportunity to reduce the sample size for a study is an important and necessary step in drug development.
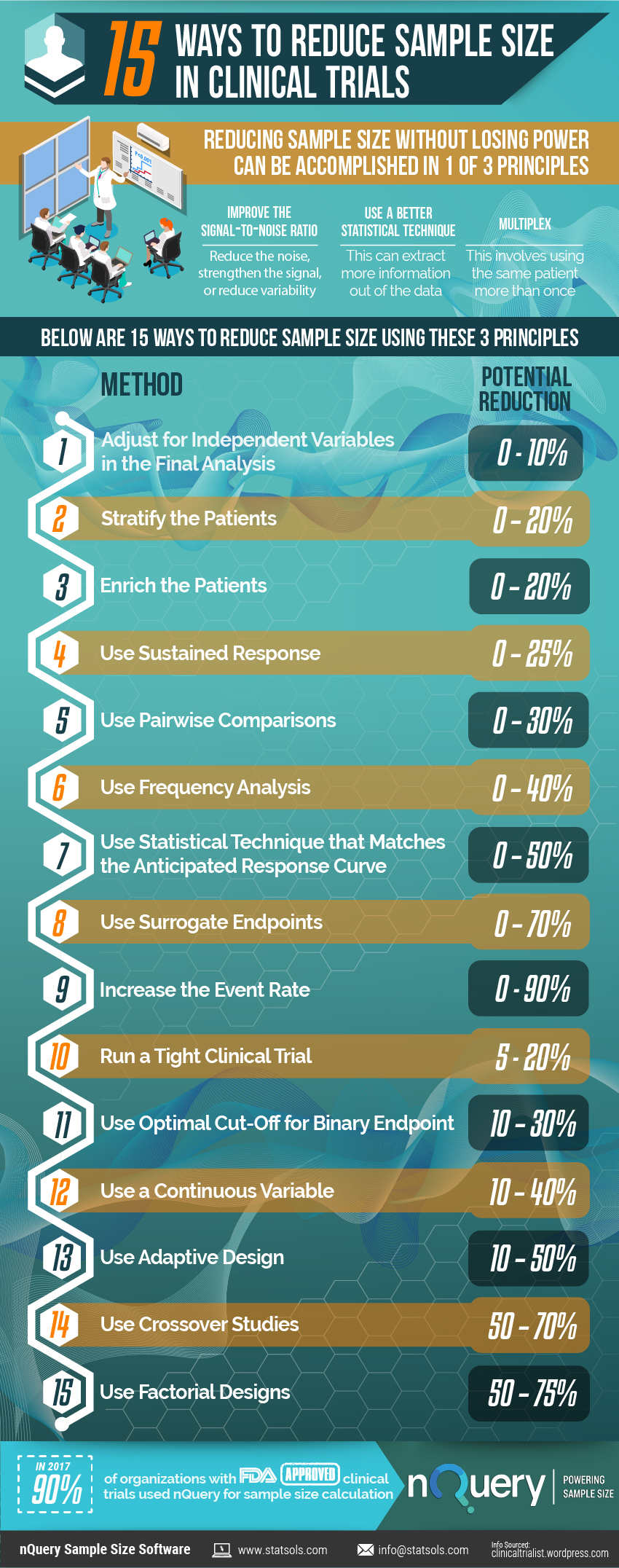
Reducing sample size without losing power can be accomplished by one of three principles.
In this infographic, we examine these and present 15 ways researchers can reduce sample size in clinical trials.
| 15 Ways to Reduce Sample Size In Clinical Trials | ||
| # | Method | Potential Reduction |
| 1 | Adjust for Independent Variables in the Final Analysis | 0 – 10% |
| 2 | Stratify the Patients | 0 – 20% |
| 3 | Enrich the Patients | 0 – 20% |
| 4 | Use Sustained Response | 0 – 25% |
| 5 | Use Pairwise Comparisons | 0 – 30% |
| 6 | Use Frequency Analysis | 0 – 40% |
| 7 | Use Statistical Technique that Matches the Anticipated Response Curve | 0 – 50% |
| 8 | Use Surrogate Endpoints | 0 – 70% |
| 9 | Increase the Event Rate | 0 – 90% |
| 10 | Run a Tight Clinical Trial | 5 – 20% |
| 11 | Use Optimal Cut-Off for Binary Endpoint | 10 – 30% |
| 12 | Use a Continuous Variable | 10 – 40% |
| 13 | Use Adaptive Design | 10 – 50% |
| 14 | Use Crossover Studies | 50 – 70% |
| 15 | Use Factorial Designs | 50 – 75% |
Take a look at the extended guide on the nQuery Sample size Software website.
Need help to design a clinical trial? Hire qualified experts on Kolabtree who can help you with data collection methods, statistical analysis and validation, and optimizing study design. It’s free to post your project and get quotes.



![[INFOGRAPHIC] 15 Ways to Reduce Sample Size In Clinical Trials](https://www.kolabtree.com/blog/wp-content/uploads/2018/11/computer-1149148_1280-1-702x336.jpg)



